Objective: This experiment aims to discover if temperature has an effect on the rates of sea urchin embryonic development. By altering the external environment, the experiment will determine if temperature will affect the length of the first and subsequent cell cycles upto the pluteus larvae stage.

Introduction:
Sea urchin embryos exhibit radial holoblastic cleavage in which the first and second cleavages are both meridional and perpendicular to each other. The third cleavage is equatorial, and the fourth cleavage divides unequally to produce mesomeres, macromeres, and micromeres (Gilbert, 2003). These cleavage patterns are well understood on a group level, but it is not completely clear how the individual cells are regulated on a molecular level. One can follow the egg from fertilization to blastula to gastrula to pluteus larvae formation, but this is usually performed under ideal situations. If the conditions change, such as the salt concentration or temperature of the water, how will this affect the development of the sea urchin?
Many processes, acting in sequence or in parallel, make up the cell cycle. Many studies have shown how the cell cycle is regulated at the molecular level in the cyclic accumulation and destruction of cyclins and the cyclic activity of M phase-promoting factor (Meijer et al., 1991). However, only some of these processes affect the length of the cycle (Nurse, 1990). Other environmental factors have been suggested as additional regulators of the cell cycle. For example, temperature has been shown to have a number of effects on the role of various phases of mitotic division, such as the rate of oxygen consumption and carbon dioxide production (Hoadley, 1937). Researchers have found that cell cycle events differ greatly in their degree of temperature dependence. Sea urchins are one organism that is affected by temperature. A temperature- dependent period exists after egg fertilization in which the duration of the cleavage cycle and normal development patterns are affected by the temperature of the surrounding environment (Yamada, K, and K. Mihashi, 1998).
Sewell and Young's experiment 'Temperature limits to fertilization and early development in the tropical sea urchin Echinometra lucunter" showed that each sea urchin species has an optimal fertilization temperature based on the average temperature found in its natural habitat (Sewell, M.A.and C.M. Young, 1999). This optimal temperature is necessary for the successful development of the embryos and pluteus larvae (Katsuyuki, Y., and K. Mihashi, 1998).
A number of species of sea urchins are found around the world. Each species thrives in their own unique environment. The subject of this experiment isLytechinus variegatus, which is found in the Gulf of Mexico and normally develops in water temperatures near 22°C.
Materials:
male and female Lytechinus variegates
100 mL beakers
sterile syringes and needles
petri dishes with covers
artificial salt water (ASW)
graduated cylinder
glass Pasteur pipets
glass depression slides
ice bucket
aluminum foil
0.5M KCl
14°C incubator
37°C incubator
thermometer
light microspcope
digital camera
Procedure:
1.Obtain gametes of sea urchins; store egg in a gently stirred suspension at 22°C, washing several times with artificial sea water (ASW)
2. Collect sperm from sea urchin and dilute one drop into 10 ml beaker containing ASW.
3. Prepare three baths of ASW at 14°C, 22°C (control), and 37° using a water bath or incubator.
4. Fertilize all the eggs with the diluted sperm in a 100 ml beaker.
5. Once fertilization envelope has developed, note time.
6. Transfer fertilized eggs and 30 ml of ASW into three glass dishes according to appropriate temperature.
7. Dilute with 30 ml of ASW at appropriate temperature (14°C, 22°C, and 38°C)
8. Cover each glass dish and place into incubator maintaining temperature.
9. Monitor the progress of development by taking a small sample and placing it on a depression slide to be viewed under a light microscope.
10. When 90% or more of the eggs have completed cytokinesis, note the time after fertilization and take still photographs.
11. Allow the fertilized embryos to develop for 24 hours.
12. After 24 hours, transfer 1 drop of each of the three samples onto depression slides.
13. Observe the sample under the light microscope. Take still photographs and compare embryonic development.
14. 48 hours after fertilization, repeat steps 12 and 13.
Results:
Temperature had an effect on the cleavage rates of developing sea urchin embryos. Generally, the higher the environmental temperature, the faster the embryo divided. At 37°C, the sea urchin eggs reached the first cell cleavage at 40 minutes after fertilization, and cleaved the second time at 65 minutes after fertilization. Abnormalities in the gastrulating embryo were observed at 37°C. Under ideal conditions, 22°C, the embryos first cleavage cycle occurred at 60 minutes after fertilization and the second cleavage cycle at 100 minutes. At 14 °C, the cleavage rate was dramatically slower, with the first division taking place at 95 minutes after fertilization. Only 50% of the embryos continued to divide, in which they reached the second cleavage at 205 minutes after fertilization. When the cultures were photographed 95 minutes after fertilization, the embryos incubated at 14°C had only divided once, the control embryos had divided twice, and the embryos incubated at 37-38°C had already divided 3 times (Figure 2).
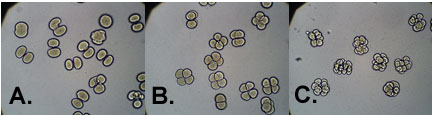
Figure 2. Sea Urchin embryos 95 minutes after fertilization. Embryos (A.) incubated at 14°C, (B.) incubated at 22°C(Control), and (C.) incubated at 38°C.
Twenty-four hours after fertilization (Day 2), the control group had developed normally. The majority of the embryos displayed archenteron formation and were motile. Not all embryos kept at 14°C had hatched by day 2. The ones that had hatched did not have archenterons and were not motile. The majority of the embryos kept at 37°C had died and lacked archenterons. The remaining embryos had developed abnormally, had not hatched and were smaller than the control group (Figure 3)
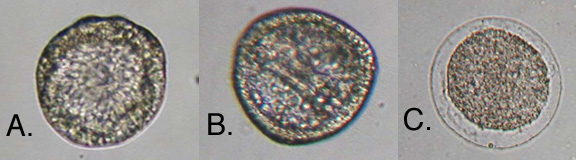
Figure 3. Sea Urchin embryos 24 hours after fertilization. Embryos (A.) incubated at 14°C, (B.) incubated at 22°C(Control), and (C.) incubated at 38°C.
Forty-eight hours after fertilization, all embryos of the control group were at the pluteus larvae stage. At 14°C, the vast majority of the embryos were dead and those alive still did not show signs of regular development such as archenteron formation. At 37°C, there were some pluteus larvae but the majority of the embryos were small and unhatched (Figure 4).
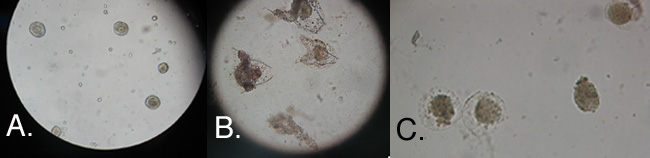
Figure 4. Sea Urchin embryos 48 hours after fertilization. Embryos (A.) incubated at 14°C, (B.) incubated at 22°C(Control), and (C.) incubated at 38°C.
Discussion:
Sea urchin development can be altered by a series of environmental changes. One of these changes is temperature. In our initial experiment, we showed that sea urchins undergo cleavage more rapidly in higher temperatures. In our second experiment observations were extended over a longer period of time. We observed that changes in temperature caused changes in development. Twenty-four hours after fertilization, archenteron formation was visible in the control embryos whereas the embryos in the other two temperatures were undergoing abnormal and slow development. Lastly, 48 hours after fertilization the control embryo were all at the pluteus larvae stage while the other embryos were either dead or developing abnormally. Overall, sea urchin embryo development is temperature- dependent, with the process occurring at a faster rate and demonstrating abnormal developments at warmer than ideal temperatures, and a slower rate, and even cell death, taking place at cooler than optimal temperatures
近日,服務科學領域的全球領導者賽默飛世爾科技(以下簡稱賽默飛)宣布,在達成收購意向兩個月之后,賽默飛以28億美元、折合人民幣約190億元的價格,完成了對TheBindingSiteGroup的全現金收......
11月15日,施普林格·自然和TheLens平臺宣布結成重要的合作伙伴關系,以更深入地揭示學術研究和數據如何能通過經濟和社會成效,加速推動創新的問題解決方式。通過將科學、投資和企業領域的開放數據更好地......
蛋白質作為構成人體組織器官的支架和主要物質,在人體生命活動中起著重要作用。蛋白質的相互作用能產生許多效應,如形成特異底物作用通道、生成新的結合位點、失活、作用底物專一性和動力學變化等,細胞的代謝、信號......
2021年9月9日,無錫臻和生物科技有限公司(以下簡稱“臻和科技”)與美國VyantBio公司簽署TissueofOrigin?(以下簡稱“TOO?”)全球權益和ZL轉讓協議,全資收購這款唯一獲FDA......
2021年7月20日,JournalofCellularPhysiology及JournalofCellularBiochemistry同時撤回了中國學者49篇文章。從2019年開始,Journalo......
6月10日,QS教育集團正式發布了2021年世界大學排名,中國共有83所高校上榜,包括內地高校51所,港澳臺地區高校32所。中國大學的總體排名情況已經連續數年呈上升趨勢,今年再度刷新了榜單。大學排名,......
磷酸甘油酸突變酶1(PGAM1)通過其代謝活性以及與其他蛋白質(例如α平滑肌肌動蛋白(ACTA2))的相互作用,在癌癥代謝和腫瘤進展中起關鍵作用。變構調節被認為是發現針對PGAM1的高選擇性和有效抑制......
BCEIA2019InternationalSummitonScientificInstrumentsDevelopment--RegistrationinprogressChinaisoneofth......
作為一種重要的植物激素,茉莉酸不僅調控植物對于機械損傷、昆蟲取食和腐生型病原菌侵害的防御反應,還參與調控諸多生長發育過程。basicHelix-Loop-Helix(bHLH)類型轉錄因子MYC2是茉......
ThePlantCell是植物領域的著名學術期刊,對植物學的發展起到了重要的引領作用。為慶祝創刊30周年,ThePlantCell雜志社邀請部分編委會成員及其他科學家對發表在該雜志的重要研究工作進行評......