B、D圖: 顯示兩組樣本外泌體CD47表達異常,乳腺癌組CD47明顯表達減少,統計學差異P值=0.004說明巨噬細胞啟動吞噬效力。
E圖:在B、D圖個選取N=60人份血液標本。 未配對t檢驗,P值<0.05.
F圖:通過ELISA 測量健康人N=40人份,乳腺癌N=50份,CD47表達量,未配對t 檢驗,P值<0.01.
實驗證明:超納米級流式無法匹敵的散射光和分辨率可以檢測不同細胞領域,通過健康人與乳腺癌患者CD47抗體表達量的不同,可以判定癌癥療效和預后指標。
參考文獻:
1. Harding, C. V., Heuser, J. E. &
Stahl, P. D. Exosomes: looking back three decades and into the future.
The Journal of cell biology 200,
367–371, doi: 10.1083/jcb.201212113 (2013).
2.
van der Pol, E. et al. Optical and non-optical methods for detection
and characterization of microparticles and exosomes. Journal of
thrombosis and haemostasis: JTH 8, 2596–2607, doi: 10.1111/j.1538-7836.2010.04074.x (2010).
3. Dragovic, R. A. et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine:
nanotechnology, biology, and medicine 7, 780–788, doi: 10.1016/j.nano.2011.04.003 (2011).
4. Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells.
Nature cell biology 9, 654–659, doi: 10.1038/ncb1596 (2007).
5. Schorey, J. S. & Bhatnagar, S. Exosome function: from tumor immunology to pathogen biology. Traffic 9, 871–881, doi:
10.1111/j.1600-0854.2008.00734.x (2008).
6.
Iero, M. et al. Tumour-released exosomes and their implications in
cancer immunity. Cell death and differentiation 15, 80–88, doi:
10.1038/sj.cdd.4402237 (2008)
微囊泡檢測
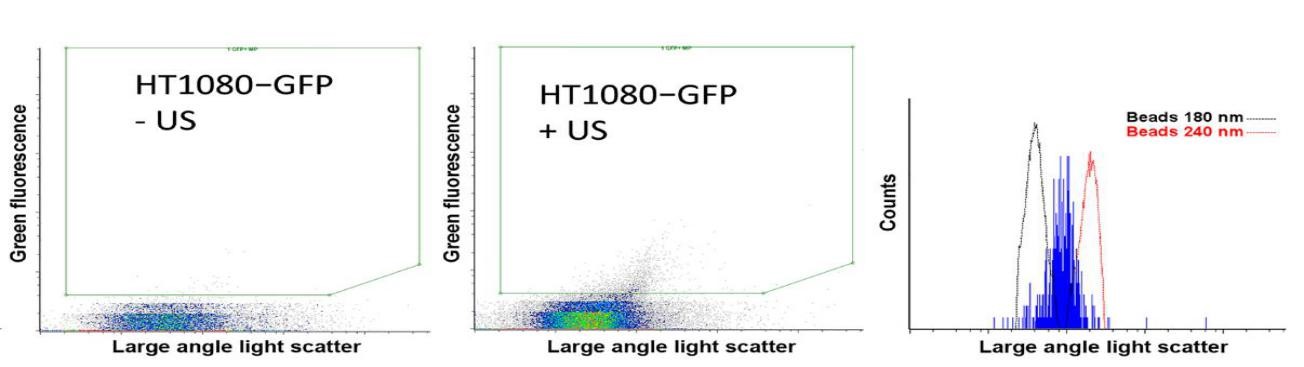
日前,加拿大科學家Paproski R J等人首次研究證實,通過使用超靈敏納米流式對胞外囊泡EVs的釋放量進行絕對計數和鑒定(如圖),發現配合納米液滴,使用超聲刺激可以顯著增強腫瘤細胞EVs的釋放,可使腫瘤細胞釋放足夠的EVs進入血液當中。同時,在這些胞外囊泡EVs中,Paproski R J和其同事成功檢測到腫瘤相關蛋白、DNA、mRNA和miRNA。該研究成果為腫瘤的無創檢測分析和遺傳表型分析提供了重大的診療價值。
參考文獻:
Paproski R J, Jovel J, Wong G K S, et al.
Enhanced detection of cancer biomarkers in blood-borne extracellular
vesicles using nanodroplets and focused ultrasound[J]. Cancer Research,
2017, 77(1): 3-13.
Apogee A50 納米級流式-外泌體/微囊泡檢測優勢大展示
亮點一:獨有的散射光靈敏度80nm及分辨率10nm,高于傳統流式10倍,最高3激光及12檢測器,并可加配自動進樣器;
亮點二:高濃度樣本(109 cell/ml)直接檢測,渾濁液亦可直接上樣;
