EXTRACTION OF LIPIDS FROM LIPOPHORIN
Since lipids are hydrophobic, they are better soluble in organic solvents than in water. Because the lipids are buried into protein particles simple shaking of the aqueous protein solution with an immiscible solvent will not lead to efficient extraction. 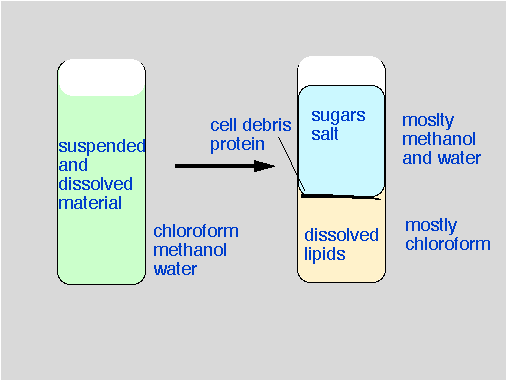 The method of choice is a one phase extraction, as pioneered by Folch and modified by Bligh and Dyer (1959). Water, methanol, and chloroform are mixed in the ratio 1:2:0.8. Under these conditions, all three solvents remain mixed, and the lipoproteins are exposed to the solvent molecules. Subsequently, water and chloroform is added to shift the ration to 2:2:1.8, now the polar and nonpolar solvents are no longer miscible, and we get two phases.
The method of choice is a one phase extraction, as pioneered by Folch and modified by Bligh and Dyer (1959). Water, methanol, and chloroform are mixed in the ratio 1:2:0.8. Under these conditions, all three solvents remain mixed, and the lipoproteins are exposed to the solvent molecules. Subsequently, water and chloroform is added to shift the ration to 2:2:1.8, now the polar and nonpolar solvents are no longer miscible, and we get two phases.
Note: Chloroform is heavier than water. The speed and ease of this method make it suitable for multiple extractions, e.g. of plasma samples, provided that a small amount of of aqueous contamination can be tolerated.
Use only glass pipettes throughout this experiment.
Always make a flow chart of the procedure prior to carrying out the experiment.

1. Retrieve the lipophorin from the freeze-dryer, weigh out the lyophilized powder and put in a small 7 ml glass vial. Close with a lid containing teflon lining. 2. To your freeze-dried lipophorin, add 0.8 ml water. Shake vigorously to suspend the lipoprotein.
3. Add 10 μl of internal lipid standard (solution contains 1 mg/ml of all standards) per mg protein (as weighted out).
4. Add chloroform and methanol, mixing in the vortex and homogenizing using a pasteur pipette after each addition so that the final proportions are now
| chloroform | methanol | water |
5. Allow to stand for 10 min at room temp.
6. Add 1 ml chloroform and 1 ml water, mixing and homogenizing as above after each addition. The final proportions are now
| chloroform | methanol | water |
7. Centrifuge in the Hermle centrifuge (in B 8220) for 5 min at 4000 rpm to form two layers. (Use large rubber adapters so that the vial can be removed without disturbing the layers). The lower, chloroform layer contains lipids. The upper layer contains water, methanol, and water soluble salts.
8. Transfer the lower chloroform containing layer (using a pasteur pipette) into clean glass vials. Make sure to prevent contamination from the upper layer or the interphase. If the transferred chloroform phase contains water (it may look cloudy), dry the solution by adding a spatula full of anhydrous sodium sulfate. Decant into a new vial, and evaporate the solvent (in the fume hood) in a stream of nitrogen.
9. You should see some yellow semi-liquid phase at the bottom of the vial. This is your lipid extract. Use the Teflon coated lids. Store at -20 °C.
蛋白質作為構成人體組織器官的支架和主要物質,在人體生命活動中起著重要作用。蛋白質的相互作用能產生許多效應,如形成特異底物作用通道、生成新的結合位點、失活、作用底物專一性和動力學變化等,細胞的代謝、信號......
2021年9月9日,無錫臻和生物科技有限公司(以下簡稱“臻和科技”)與美國VyantBio公司簽署TissueofOrigin?(以下簡稱“TOO?”)全球權益和ZL轉讓協議,全資收購這款唯一獲FDA......
2021年7月20日,JournalofCellularPhysiology及JournalofCellularBiochemistry同時撤回了中國學者49篇文章。從2019年開始,Journalo......
磷酸甘油酸突變酶1(PGAM1)通過其代謝活性以及與其他蛋白質(例如α平滑肌肌動蛋白(ACTA2))的相互作用,在癌癥代謝和腫瘤進展中起關鍵作用。變構調節被認為是發現針對PGAM1的高選擇性和有效抑制......
2018年12月6日,來自圣迭戈的消息——Illumina公司(納斯達克股票代碼:ILMN)今天宣布推出新型高密度基因分型芯片Infinium?GlobalDiversityArray。這款芯片設計源......
分析測試百科網訊2015年8月16日,中國儀器儀表學會分析儀器分會樣品制備專業委員主辦的第二屆全國樣品制備學術報告會在貴陽舉行。本次大會與中國儀器儀表學會分析儀器分會2015學術年會同期舉辦,參會20......
SDS-PAGE異常電泳現象及分析SDS-PAGEHallofShame.pdf 很不錯的東東~~推薦下~......
今天在網上淘到的一本書GeneFlowfromGMPlants,05年,還算比較新。望大家多多支持。GeneFlowfromGMPlantsEditedbyGUYM.POPPYSchoolofBiol......
1)TheyeastspeciesSaccharomycescerevisiaewereculturedinSDmediawith2%raffinose.Beforeextraction,yeastw......
Preparationofdenaturing6%polyacrylamidegelsformicrosatelliteanalysis(alsoforSSAP,high-resolutionIRAP......