Background:
Zebrafish,or the teleost fish Danio rerio,is a rapidly developing organism that is a
popular species for studying vertebrate development. Cleavage in the Zebrafish only occurs in
the blastodisc,a region of cytoplasm in the animal cap of the egg. This type of meroblastic
cleavage is called discoidal. Cell divisions are rapid in the zebrafish,with divisions taking
about fifteen minutes each. Gastrulation is usually complete after a little over ten hours from
fertilization. About 24 hours after fertilization,the embryo has formed most of its tissue and
organ primordia and displays the tadpole like form.
This experiment will look at the development of motor neurons in zebrafish at the 24
hour stage. Motor neurons arise from neurons at the ventrolateral margin of the neural tube.
These cells are first signaled by Sonic hedgehog from the notocord to instruct them to become
ventral neurons,and later instructed by Sonic hedgehog from the floor plate cells to become
motor neurons instead of an interneuron. Further motor neuron specification is required,
since they must innervate all the various parts of the body. In mammals,motor neurons are
grouped into three large columns according to their target. Motor neurons in the column of Terni
(CT) project into the sympathetic ganglia,those in the lateral motor column (LMC) extend to
the limb musculature,and those of the medial motor columns (MMC) project to the axial
muscles. The migration patterns and proliferation of these motor neurons is thought to be
regulated by the cell's age when it last divides.
In addition,the development of somites in a 24 hour old zebrafish will also be
examined. Somites give rise to the cells that form the vertebrae,ribs and the skeletal muscles
of the back. They determine the migration pathways of neural crest cells and spinal nerve axons.
They first appear in the anterior portion of the trunk,and form as a function of the
developmental rate of the embryo. Therefore,it is possible to use the number of somites
present to gauge how far the embryo has developed.
To examine how thoroughly the motor neurons and somites have developed by the 24
hour stage,the technique of whole mount antibody staining will be used. The ZNP-1 antibody
will specifically bind to the primary motor neurons. Since ZNP-1 will not bind to the somites,
another antibody,F6,will be used to bind to the somite boundaries. The ZNP-1 and F6
treated embryos will be treated with a secondary antibody, Fluorescent Goat anti-mouse IgG,
so that the stained neurons and somites can be observed under a fluorescent microscope.
Procedure:
1. Dechorionate 24 hour zebrafish embryos in the pharyngula stage with 2 fine forceps.
2. Fix approximately of the embryos with 4%, and the other half in 1% paraformaldehyde in PBS for one hour.
3. Wash embryos 3x in 5 ml PBS to remove fixative
4. Incubate embryos in PBS with goat serum and 0.2% saponin
5a. Add the primary antibody,ZNP-1 at 1/2000 to half the cells with 4% paraformaldehyde,keep the other half as a control,incubate for 24 hours.
5b. Add the primary antibody,F6 at 1/500 to half the cells with 1% paraformaldehyde,keep the other half as a control,incubate for 24 hours.
6. Wash embryos with several changes of 5 ml PBS to remove antibody
7. Incubate with the secondary antibody,Fluorescent Goat anti-mouse IgG+ for 45 minutes
8. Wash with several changes of PBS
9. Mount with depression slides and observe stained embryos using the fluorescent microscope.
10. Look for individual brightly stained cells in the tail region.
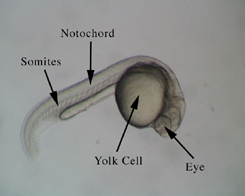
Figure 1.
Dechorionated Zebrafish embryo at approximately 24 hours
Results
All four groups of embryos were observed under a fluorescent microscope. The trunk region was observed for areas of brighter cells. In picture A, vertical lines of cells are slightly brighter than surrounding cells. This is where the motor neurons will develop. In picture C, we see evidence of a row of somites developing along the trunk region. The controls show less evidence of developing motor neurons or somites.
Figure 2.
Pictures of fixed zebrafish embryos under a fluorescent microscope
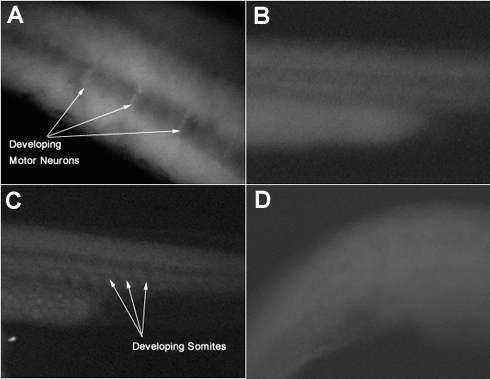
A-Trunk region of a 24 hour zebrafish embryo treated with motor neuron specific ZNP-1 antibodies
B-Trunk region of a control, unstained 24 hour zebrafish embryo
C-Trunk region of a 24 hour zebrafish embryo treated with somite boundary specific F6 antibodies
D-Trunk region of a control, unstained 24 hour zebrafish embryo
Discussion and Conclusion
The staining of the motor neurons and the somites in the developing zebrafish is a difficult task. The zebrafish is a quickly developing organism, usually resembling the adult zebrafish after one day. The antibody for the motor neurons, will theoretically bind specifically to the developing motor neuron cells in the tail. However,given the small number of cells which are present at a given time, the resulting embryo does not show clear signs of the stained cells. In addition, a bigger factor may be the efficiency of the auto-fluorescent secondary antibody used. The cells of the zebrafish embryo often exhibit some auto fluorescence without any stimulation, so it may be difficult to differentiate the stained motor neurons from the surrounding cells, which also appear brightly stained.
The somites are also located in the tail region. They will eventually give rise to the vertebrae and the skeletal muscles in the back. The cell specific F6 antibody was used to stain the embryo for somite boundaries. However, little staining is observable because of the relatively similar appearance between the stained somite cells and surrounding cells, due to the secondary antibody resulting in stained cells which resembled surrounding cells.
The use of ZNP-1 and F6 coupled with the secondary antibody. Fluorescent Goat anti-mouse IgG+, to specifically stain the motor neurons and somites were not as successful as expected. The stain only resulted in few cells exhibiting a somewhat brighter color in the tail region,and was not conclusive. An alternate procedure using a horseradish peroxidase-conjugated secondary antibody, although more time consuming, resulted in a better signal-to-noise ratio andclearer staining.
萬物蓬勃的7月里迎來了2022年ANTOP獎的申報和評審工作。由島津企業管理(中國)有限公司申報的“3CoinONE全新體驗氣袋進樣器”ANTOP獎進入專家評審階段。獎項名稱:3CoinONE全新體驗......
青島青源峰達太赫茲科技有限公司研發團隊在國際頂級期刊《TrendsinBiotechnology》(譯名:《生物技術趨勢》)在線發表題為“THzmedicalimaging:frominvitroto......
蛋白質作為構成人體組織器官的支架和主要物質,在人體生命活動中起著重要作用。蛋白質的相互作用能產生許多效應,如形成特異底物作用通道、生成新的結合位點、失活、作用底物專一性和動力學變化等,細胞的代謝、信號......
2021年9月9日,無錫臻和生物科技有限公司(以下簡稱“臻和科技”)與美國VyantBio公司簽署TissueofOrigin?(以下簡稱“TOO?”)全球權益和專利轉讓協議,全資收購這款唯一獲FDA......
2021年7月20日,JournalofCellularPhysiology及JournalofCellularBiochemistry同時撤回了中國學者49篇文章。從2019年開始,Journalo......
安進宣布,美國FDA授予其在研firstinclass單抗bemarituzumab突破性療法認定,與改良FOLFOX6化療方案(亞葉酸鈣、氟尿嘧啶和奧沙利鉑)聯用,一線治療FGFR2b過表達和HER......
2007年,中國科學院金屬研究所研究員張志東在英國《哲學雜志》(PhilosophicalMagazine)上發表論文,提出兩個猜想,并在猜想基礎上推定出三維伊辛模型的精確解。被《哲學雜志》審稿人評價......
磷酸甘油酸突變酶1(PGAM1)通過其代謝活性以及與其他蛋白質(例如α平滑肌肌動蛋白(ACTA2))的相互作用,在癌癥代謝和腫瘤進展中起關鍵作用。變構調節被認為是發現針對PGAM1的高選擇性和有效抑制......
磷酸甘油酸突變酶1(PGAM1)通過其代謝活性以及與其他蛋白質(例如α平滑肌肌動蛋白(ACTA2))的相互作用,在癌癥代謝和腫瘤進展中起關鍵作用。變構調節被認為是發現針對PGAM1的高選擇性和有效抑制......
BCEIA2019InternationalSummitonScientificInstrumentsDevelopment--RegistrationinprogressChinaisoneofth......